The Study on Prevalence of Gastrointestinal Helminths in Chickens, Bale Zone, Dalomana District, Southeast Ethiopia
Sufian Abdo Jilo1*, Sadik Zakir Abadura1, and Sureshkumar P. Nair2
- School of Veterinary Medicine, Jimma University College of Agriculture and Veterinary Medicine, Ethiopia
- Department of Biomedical Sciences, Institute of Health, Jimma University, Ethiopia
* Corresponding author: Sufian Abdo Jilo, School of Veterinary Medicine, Jimma University College of Agriculture and Veterinary Medicine, Ethiopia. Email: sufianabdojilo@gmail.com
A B S T R A C T
Gastrointestinal Tract (GIT) helminthiasis of poultry is defined as a parasitic infection of gastrointestinal parts of poultry by macroparasite which is classified as tapeworm (cestodes), roundworm (nematodes), and flukeworm (trematodes). The common intestinal helminthic infections in local chickens include cestodes and nematodes, which may cause considerable damage such as nutritional loss, and high economic loss to the poor farmers of the rural area. A cross-sectional study on gastrointestinal helminths was conducted on 144 local breeds of chickens raised under a traditional management system in seven kebeles located around Dalomana town of Bale zone, Ethiopia. Of these chickens, 131 (91%) were infected with one of the five different helminth parasites and 13 (9%) were free of helminth parasites. The results of the current study indicated that 131 (91%) and 107 (74.3%) of the examined chickens were invariably infected by diverse species of cestodes and nematodes species, respectively. The major cestode species recovered from chickens were Raillietina echinobothrida 109 (75.5%), Raillietina tetragona 106 (73.6%), Davainea proglottina 16 (11.1%). The major nematode species encountered were Heterakis gallinarum 54 (37.5%), Ascaridia galli 51(35.4%), Capillaria anatis 10 (6.9%), Capillaria obsignata 8 (5.6%), and Capillaria annulata 7 (4.9%). The study also tried to see the prevalence of these parasites in relation to age, sex, and kebele, however, no significant difference is not indicated regarding these risk factors. This study strongly suggested that helminthiasis is a very serious problem of backyard chickens in Dalomana district, Bale zone of Oromia and appropriate control strategies need to be devised.
Introduction
Poultry includes all domestic birds kept for human food production (meat and eggs) such as chickens, turkeys, ducks, geese, ostrich, guinea fowl, and doves and pigeons. In Ethiopia, ostrich, ducks, guinea fowls, doves, and pigeons are found in their natural habitat (wild) whereas, geese and turkey are exceptionally not common in the country. Lack of access to productive and adaptable chicken breeds still remains one of the most critical challenges to increasing the economic contribution of the poultry production sector. Most of the chickens kept by smallholder farmers are unimproved indigenous flocks, well-adapted to the local environments, however having slow growth rates and very poor egg productivity1.
In Ethiopia, the total poultry population is estimated to be about 44.89 million. By the report, poultry entail cocks, cockerels, pullets, laying hens, non-laying hens, and chicks. With regard to breed, 96.46%, 0.57%, and 2.97% of the total poultry were reported to be indigenous, hybrid, and exotic ,respectively2.
The poultry industry occupies an crucial position in the provision of animal protein (meat and egg) to man and generally plays a vital role in the national economy as a revenue provider. Poultry production in Africa and parts of Asia is still distinctively divided into commercialized and village enterprise subsector3. The poultry production system in Ethiopia is an indigenous and integral part of the farming system that ranges from nil input traditional free ranges to modern production systems using relatively advanced technology. There is also a small-scale intensive system with a small number of birds (from 50 to 500) as an urban and peri-urban small-scale commercial system using exotic birds and relatively improved feeding, housing, and health care4.
Backyard poultry is usually carrying high levels of worms in their digestive systems, putting increased stress on the animal and its ability to convert feed into proteins. Anthelmintic medications (de-wormers) are cheap and effective and are beneficial even at this level of poultry production as they improve the bird’s appetite: the better the bird eats, the better its health and the greater its resilience to other diseases. Combined with vaccinations, this level of intervention can have profound impacts on family nutrition for backyard ‘hanging in’ farming households3.
Parasites are among the infectious agents that cause an alarming problem to the industry, posing adverse economic effects. Gastrointestinal parasitism leads to significant economic losses in poultry, especially in backyard poultry production4. Nematodes cause more serious problems in backyard flocks, in developing countries such as Ethiopia. The backyard scavenging production system exposes chickens to certain eggs and larvae of parasites from ingested soils and insects5. Helminth infections in the rural free-range chicken are ubiquitous and may result in subclinical diseases even when they occur in lower numbers6.
Recently, poultry farms in Ethiopia are dramatically increasing in number. However, research findings conducted in different parts of the country incriminated helminths as major causes of ill health and loss of productivity in local chickens7.
Helminth’s parasites involving nematodes (roundworms), trematodes (flatworms), and cestodes (tapeworms) affecting scavenging chickens have been widely reported, with mixed infection being very common8. In Africa, prevalence (usually of multiple infections) of up to 99% has been reported9.
There are currently a few formations in Ethiopia that show the prevalence and distribution of Gastrointestinal Tract (GIT) helminths. Different prevalence of helminth in different parts of the country According to reports, the prevalence of GIT helminths in Ethiopia reaches from 44. 5%10 to 91.01%11,12. However, it is limited by its coverage (region) of Ethiopia so that does not indicate the whole picture of the prevalence in Ethiopia. There are only a few studies conducted in central12 and northern parts of the country. A study by Mebrahtu in 2019 reported that the prevalence of GIT helminths is 90.6 % in Mekele Ethiopia7. Hence, the current study intended to estimate the prevalence and potential risk factors for the occurrence of GIT helminths in scavenging chickens in selected rural villages around Dalomana town. In order to design effective preventive and control strategies, it is very much essential to know about the available helminth parasite species and their burden on chickens in the study area. Therefore, the present study was conducted under backyard management systems, to determine the prevalence of gastrointestinal helminth parasites and identify the parasite species that infect local breed chicken in the study area.
Materials ad Methods
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board of Jimma University, College of Agriculture and Veterinary Medicine. Furthermore, verbal informed consent was obtained from restaurant owners and household individuals participated in the study, after explaining the purpose of the study in their local language (Afan Oromo).
Study area
The study was conducted in Dalomana district, Bale Zone, Southeastern Oromia, Ethiopia. The altitude of the study area ranges from 850 to 2800 m. The area experiences a bimodal rainfall occurring from September to November and March to June. Annual rainfall ranges from 700 to 1200 mm. The mean annual temperature is 29.5˚C while the annual minimum temperature is 21˚C and maximum is 38˚C and the mean annual rainfall is 986.2 mm13.
Study design
A cross-sectional survey was conducted from November 2020 to March 2021 to provide baseline information on the prevalence and distribution of GIT helminths of scavenging chickens rearing under the traditional system in Dalomana district, southeast Ethiopia.
Study population and management
The local breed of Chickens under backyard production systems in and around Dalomana town, were considered during the study. The chickens used in this study were those slaughtered in volunteer restaurants and households with backyard management for postmortem examination chickens between the age of 6 and 12 months taken as young and chicken greater than 1 year taken as adults following the method used by Magwisha et al6 with some modifications.
Sample size and sampling methods
The sample size was calculated according to Thrusfield14. The previous study by Eshetu et al.11 indicated 91.01% prevalence with comparable agroecology and this was taken as expected prevalence. With a desired absolute precision of 5 and 95% level of confidence, sample sizes of at least 120 chickens were required. About 144 chickens were obtained from Mena 01, Mena 02, Wabaro, Chiri, Nanigadera, Hayaoda, and Irba village, in a different hotel and from the individual houses during the festival.
Examination of chickens for the type of worms
The chickens were slaughtered at a different hotel and the GIT was obtained. The GIT was then separated into the esophagus, crop, proventriculus, gizzard, small intestine, and caeca. Each part was opened and its contents were emptied separately into labeled beakers. Then contents were washed into a Petri dish and examined under a binocular stereo turret microscope with a Halogen bulb (Olympus, Japan). The larger helminths were collected directly and smaller ones were isolated under the stereo turret microscope. Worms were grouped and counted before being stored in plastic bottles containing 70% alcohol according to a method described by Ashenafi and Eshetu12.
Identification of worms
Identification of collected helminths was done at the Laboratory of Dalomana veterinary clinic. All helminths were identified by hand lens and under a stereo, turret microscope using helminthological keys of Calneket al.15 and Ashenafi and Eshetu12. with the following steps
1) The gastrointestinal tracts were collected into plastic bags and taken to the diagnostic and examination laboratory.
2) After proper labeling and dating, they should be sent immediately to the processing laboratory.
3) Samples that could not be immediately analyzed were stored in the refrigerator.
4) The gastrointestinal tracts were separated into gizzard, crop, small intestine, large intestine, and caecum after which each region was cut open by longitudinal incision also was cut open by longitudinal incision.
5) Intestinal scrapping was done and any parasite seen was removed with forceps, washed in saline, and identified.
6) All worms visible to the naked eye were removed using thumb forceps.
7) All the adult worms were identified directly under the stereo turret microscope.
8) Scrapings were also taken from the mucosae of the upper, middle, and lower intestine and caecum, and examined under the stereo turret microscope.
9) Collect mucous exudate and deep mucosal scrapings and press into a thin layer between two thick pieces of plate glass. Examine under stereo turret microscope magnification for the presence of parasites. The Cappilaria eggs of the parasite are under magnification, look for bipolar, lemon-shaped eggs in the female capillaries, distinctive characteristics of tapeworms is demonstrated by examining the scolex, eggs and individual proglottids of recently shed, and whole live specimens. General
morphology used in the identification of the nematodes is a body covering, mouth, sex organ, and others. Ascaridia galli types of nematodes are large, thick, yellowish-white worms; their head has three large lips. The male is 50–76 mm long and 1.21 mm wide; the perianal sucker is oval or circular, with a strong chitinous wall with a papilliform interruption on its posterior rim; the tail has narrow caudal alae or membranes and 10 pairs of papillae; the first pair of ventral caudal papillae is anterior to the perianal sucker; the fourth pair are widely separated, compare with Ascaridia dissimilis; and spicules are nearly equal and narrow, with blunt ends and slight indentations. The larger female is 60–116 mm long and 1.8 mm wide; the vulva is in the anterior part of the body, and the eggs are elliptical, thick-shelled, and not embryonated at the time of deposition.
Statistical analysis
The data obtained from postmortem examinations have entered into a Microsoft Excel spreadsheet; raw data were coded and then analyzed using SPSS (version 23). Descriptive analysis was used to determine the frequency and percentage of the parasite infections. Age, sex, and origin were examined with the prevalence of GIT helminths, by chi-square (χ2). The Duncan test was chosen to find the significant differences between the results. p value lesser than 0.05 was considered as the significant level.
Results
Overall helminth prevalence
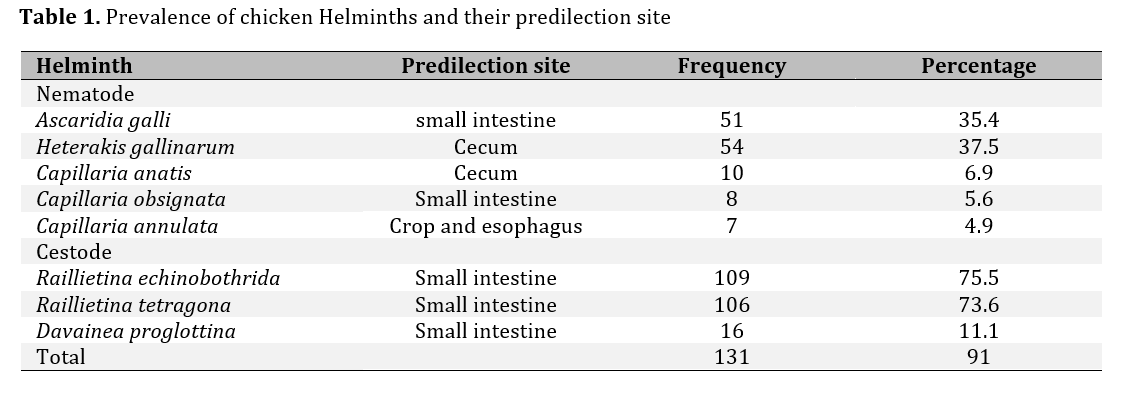
Out of the total 144 examined chickens, 91% (131/144) were found to be infected or infested with GIT helminths of many species. Analysis of data for the prevalence of the different species of helminth parasite out of the total number of affected chickens examined indicated the highest proportion for Railathenia echinobothrida 109 (75.5%) followed by Railathinia tetragona 106 (73.6%), Heterakis gallinrum 54 (37.5%), Ascarid galli 51 (35.4%), Cappilaria anatis 10 (6.9%), Cappilaria obsignata 8 (5.6%) and Capillaria annulata 7(4.9%, Table 1).
Prevalence of cestode and nematode of examined chickens
A total of 144 chickens were examined out of which 70 (64%) were females and 74 (67%) were males. The results indicated that 131 (91%) of the chickens were infected by helminths parasites and 8helminths species included5 nematodes and 3 cestodes were covered. Of the 144 chickens slaughtered and examined, 90 (62.5%) and 17 (11.8%) had single and mixed nematode infections and 38 (26.4%) and 93 (64.6%) had single and mixed cestode infection, respectively. The sites with double or triple nematodes infections were the intestinal tracts, proventriculus, and Caeca (Table 2).